
About Us
Rushi Pharma is a premier global business partner with an established brand identity in the Bulk drug (API) and Pharma Business. Our aim is to provide Consistent high quality product sourcings & Services with effective benefits to our valued Customers.
People behind Rushi Pharma carry it expertise since 1997, specializing in Bulk drug & Pharma intermediates, API's, CRO / CDMO, specialty chemicals, Enzymes, R&D chemicals, Agro & crop sciences intermediates & Agro chemicals from manufactures & Business partners.
Rushi Pharma is driven by dynamic young professionals carrying specialized expertise in the field of Pharma Business, with zeal and commitment to customer service with a realistic approach towards marketing.
 Read More
Read More
Our Services
Indenting - Direct Imports
Rushi pharma has got its reliable and trustworthy Trading partners, R&D and Manufacturing partners across the globe whom we represent in the Indian market. China is our biggest strength in Imports.
Read MoreDomestic
We provide local sources as import substitute for our clients with the following support.
- Alternative to China
- Document support for Regulated market
- Audit Compliance as per WHO GMP / FDA
- Stable supplies and assured quality
- Transparency in communication for the Client directly with the manufacturer
Import Export / Licences
- We buy excisable goods from the indian manufacturers and export to our various clients from our premises.
- We also handle exports done by indian manufacturer directly to our overseas clients.
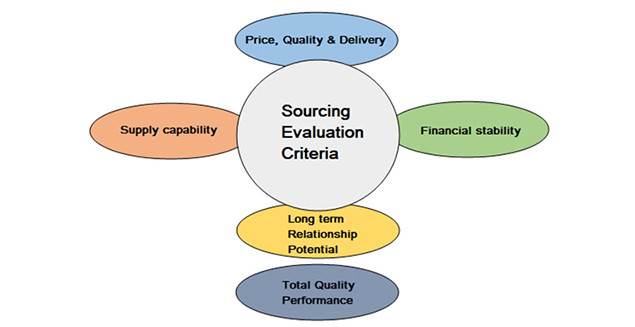
Sourcing Evaluation
Sourcing evaluation is done thoroughly as below
- Visit/ Verification of the real manufacturing site and coordinates.
- Product license/ Certifications available to the manufacturing site.
- Sourcing product status: campaign/ regular/ made against order & in-house standard specs/ MOA.
- Check whether they have basic cGMP systems.
Contract Research / Contract Development / Contract Manufacturing(APIs & Intermediates)
- Company has got it's contract R&D facility available in Hyderabad with strength of 5 Ph.D.'s, 30 Scientists, having 16 years post doc experience in USA.
- Working in teams with a team leader for each project and can handle multiple projects at any give point of time. . .
Formulations CRO
Company can offer below services in Finished Dosage Formulations [FDF]
- Formulation Development
- Formulation Tech Packages
- Formulation Tech Transfers
- Outright sale of ANDA's.
Enzyme Technology
Rushi Pharma has got it's expertise in consulting and can provide the below services:
- Enzyme Technology for commercial Intermediates and API's.
- Industrial Enzymes
- Bio Pigments
- Fermentation process development
- Fermenter design
- Solid state fermentation.
RLD Sourcing (Reference listed drugs sourcing)
Rushi pharma can do RLD sourcing upon requirements and enquiries given
Read MoreAnalytical
We have close cooperation with various Analytical Labs in hyderabad, India for the following and can assist in getting the below services done.
- Analytical (Chemical, Instrumental & Microbiological) Complete analysis or Individual test analysis of Active pharma Ingredients (Bulk Drugs), Injectables, Syrups, Ointments, Tablets, Capsules, Excipients, Raw Materials, Packing Material intermediates and In-process Samples as per Pharmacopoeia or Customer Testing Procedures
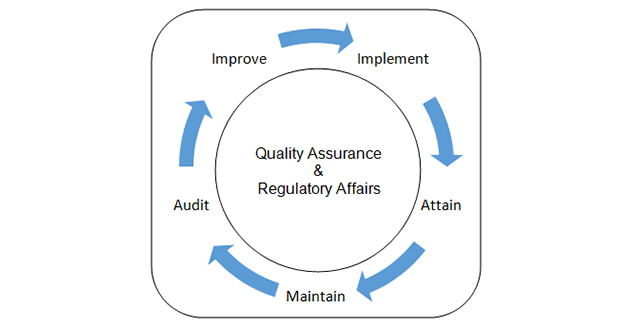
Quality / Regulatory
We are closely associated with a Regulatory service support partner to offer Quality/Regulatory Services. Listed below are some of them for reference.
- Review of NCEs, NMEs Chemistry and Manufacturing Controls (CMC)
- Design, Review and Qualification of Mfg., Laboratory, Packaging facilities
- Remediation of existing facilities to bring in compliance with QA/RA.
Knowledge Base
- D-U-N-S: 854321383
- LEI: 9845004484A1C4C82E71
- MSME UDYAM-TS-02-0059615











 Indenting - Direct Imports
Indenting - Direct Imports Domestic
Domestic Import / Export / Licences
Import / Export / Licences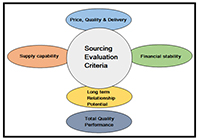 Sourcing Evaluation
Sourcing Evaluation CRO / CDMO (API & Intermediates)
CRO / CDMO (API & Intermediates) Formulations CRO
Formulations CRO Enzyme Technology
Enzyme Technology RLD Sourcing
RLD Sourcing Analytical
Analytical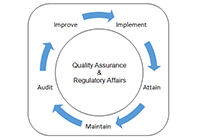 Quality / Regulatory
Quality / Regulatory